In modern covid times, we’re all very aware of how diseases can spread across borders through international air travel. Something similar could be happening with bird diseases and our kākāpõ, takahē and other endangered species on their remote sanctuary islands. ‘Air travellers’ – in this case visiting seabirds – could be bringing avian diseases with them when they come into land.

Researchers Manjula Jayasinghe, Anne Midwinter, Wendi Roe, Emilie Vallee, Charlotte Bolwell and Brett Gartrell from the School of Veterinary Science, Massey University, have been looking at one bacterial disease, erysipelas, known to have killed kākāpō, takahē and kiwi in the past. Is the bacteria present in some of the seabird species that share their island sanctuary – and if so, how might it be transmitted between such very different species?
Firstly, what exactly is erysipelas?
“Erysipelas is the infectious disease caused by the bacterium Erysipelothrix rhusiopathiae in a wide range of mammals, birds, some reptiles, and fish. This gram-positive bacillus is ubiquitous in nature and considered to be an opportunistic pathogen. The organism can be found in the tonsils or the intestinal tract of healthy carrier animals,” write the authors, in a paper published in the Journal of Wildlife Diseases.
Not only does it infect a wide range of animals, the bacterium can also survive in soil for up to 35 days and multiplies in the soil during warm weather. It’s believed to be transmitted through the gastrointestinal tract after ingestion of contaminated food or water, or through breaks in the mucous membranes or skin. Carrier animals, without symptoms, may then spread it through the environment and on to other susceptible animals.
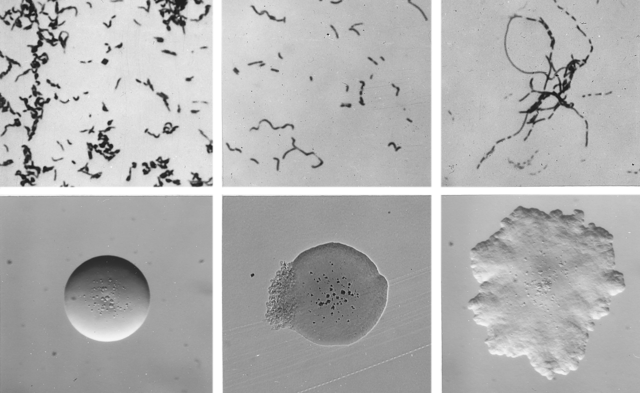
“Although the main route of shedding has not been established, E. rhusiopathiae might enter the environment through infected carcasses and through saliva and droppings of live birds. Erysipelothrix rhusiopathiae has been isolated most frequently from the cecal tonsils, liver, large intestine, heart, and blood of carrier birds. Erysipelas in birds is characterized by either acute, fulminating infections or, more rarely, chronic infections causing infertility in male birds and reduced egg production in female birds.”
So even if it doesn’t kill our endangered bird species, an infection could make the males infertile and decrease the number of eggs laid by females – not good for species conservation! So how much of a problem is erysipelas?
“In July 2004, an outbreak of erysipelas affected the success of a translocation of juvenile kākāpō from Whenua Hou (Codfish Island) to Chalky Island, killing 3/19 birds translocated. This was the first time that an infectious disease had affected a translocation event in kākāpō and the first time that erysipelas was reported in this critically endangered New Zealand parrot. Subsequently, sporadic deaths from erysipelas have been seen in takahē, kākāpō from two islands studied, and kiwi from another location, indicating the persistence of this bacterium in New Zealand ecosystems.”
Why are seabirds suspect?
“Many species of wild birds and mammals in various geographic regions have been reported to carry E. rhusiopathiae and act as reservoirs in natural ecosystems, but the prevalence in wild bird populations is unknown. During the erysipelas outbreak in 2004, seabirds were considered the most likely source of infection for the kākāpō, as both the source and the destination islands have a diverse fauna of marine and terrestrial birds, and 10 of 15 samples of ulnar bone marrow from seabird carcasses recovered from Whenua Hou at the time of the kākāpō mortalities were culture-positive for E. rhusiopathiae.”
For this latest research project, study samples were collected from two predator-free offshore islands located approximately 1,000 kilometres apart: Te Hauturu-o-Toi (Little Barrier Island) and Whenua Hou.

Te Hauturu-o-Toi is a nature reserve with an area of 28 square kilometres, 80 km to the north of Auckland.
“It is a highly eroded, extinct volcanic island, and a series of steep ridges radiating from a central range toward the coast have deeply dissected the land. An altitudinal gradient can be seen in the forest, and the forest cover in higher altitude areas is considered intact. It is a refuge for more than 350 species of plants and several species of threatened New Zealand birds. Saddleback and kākāpō are two of the endemic bird species that have been translocated to this island.”
It’s also a popular breeding ground for seabirds.
“Te Hauturu-o-Toi is the most important breeding ground for the endangered Cook’s petrel, with more than 50,000 breeding pairs in higher-altitude areas. Other burrowing seabird species found on the island include the black petrel and the grey faced petrel. A population of critically endangered New Zealand storm petrels was identified breeding on the island in 2003. Additionally, little blue penguin breed in the forest areas.”
At the opposite end of Aotearoa, lies Whenua Hou.
“Whenua Hou has a land area of 14 square kilometres and is situated in southern New Zealand, 3 km west from the northwest coast of Rakiura (Stewart Island). This nature reserve was identified as a potential location for native species in 1960, and measures were taken for island restoration, including a restriction on unauthorized access, in 1968. The summit of the island is close to the south coastal area and reaches a height of 250 metres. A large valley in the northeast side and steep coastal cliffs on the southwest, are the predominant features. It is home to southern short-tailed bats, kākā, fernbirds, red-crowned parakeet, yellow-crowned parakeet, pacific black ducks and a breeding population of kākāpō.”
Whenua Hou also provides a home for translocated populations of endangered mōhua and Campbell Island teal.

“It is important as a breeding ground for several species of seabirds, including endemic species. Common diving petrels, South Georgian diving petrels, Cook’s petrels, mottled petrels and sooty shearwaters are the burrowing seabirds found on this island. Diving petrel and prions are limited to areas near the coast, whereas burrows of forest-nesting species, such as Cook’s petrel and mottled petrel, are distributed across large areas of slope and ridge-top habitats, mostly at higher altitudes.”
So there’s no shortage of suspects who could have flown to either island carrying a cargo of infectious bacteria. For their investigation, the researchers sampled dead and live seabirds on both islands during spring and early summer of 2018, when the seabirds had returned for breeding but had not yet started incubating eggs or raising chicks.
“On both islands, dead sea birds were sampled from seabird colonies and along the tracks outside the colonies. On Whenua Hou, large mottled petrel colonies sampled were distributed near the summit area (250 m). Cook’s petrel colonies on this island have a patchy distribution within the forest area. Sooty shearwater colonies were found near the coastal side on the southwest and eastern sides of the island. Some of the sooty shearwater colonies were observed closer to the Cook’s Petrel colonies. On Te Hauturu-o-Toi, dead birds were sampled from two low-altitude colonies, two high-altitude colonies, and along the tracks outside the colony areas. Black petrel colonies were observed a few metres above the higher-altitude Cook’s petrel colonies.”
For live-bird sampling adult birds were caught inside their burrows and restrained by controlling their head, wings, and feet; thick gloves and towels were used for handling.
“Birds were checked for identification bands and 0.2–0.3 mL of blood was collected from the cutaneous ulnar vein of each bird. If the birds were not banded, they were microchipped.”
A total of 50 blood samples were collected from mottled petrels on Whenua Hou. On Te Hauturu-o-Toi, 18 blood samples were collected from Cook’s petrels.

Eighty-six dead seabirds were collected on Whenua Hou over a three-week period.
“Most of the birds sampled were Mottled Petrels (36/86), followed by Sooty Shearwaters (34/86) and Cook’s Petrels (4/86). Twelve of the 86 carcasses found were extremely decomposed and could not be identified. On Te Hauturu-o-Toi, 44 dead seabirds were collected during the study period, mainly Cook’s Petrels (37/44), with single samples each of a Black Petrel, a Little Blue Penguin, and an Australasian Gannet. Four carcasses from Te Hauturu-o-Toi could not be identified.”
“E. rhusiopathiae was identified from three of the 86 dead seabirds on Whenua Hou and five of the 44 dead birds on Te Hauturu-o-Toi. On Whenua Hou, positive samples were found in two Sooty Shearwaters (2/34) and a Mottled Petrel (1/36). On Te Hauturu-o-Toi, all the birds found to be positive for E. rhusiopathiae were Cook’s Petrels (5/37). In total, eight seabirds belonging to three species were infected with this bacterium in both study locations.”
So visiting seabirds could indeed be bringing bacterial disease to these island sanctuaries – and some of those seabird species are very widely travelled.
“Our study confirmed that burrowing seabirds inhabiting two of the offshore islands used for the conservation of kākāpō and other species are a possible source of E. rhusiopathiae within these island environments. Although the prevalence of this bacterium in dead seabirds on Te Hauturu-o-Toi (11.4%) was comparatively higher than the overall prevalence on Whenua Hou (3.5%), E. rhusiopathiae was isolated from two seabird species on Whenua Hou, including the numerous sooty shearwaters.”
Sooty shearwaters, also known as tītī or muttonbirds, are a human food source in Aotearoa – and that could also be a concern.
“Sooty Shearwaters are one of the most widely distributed seabirds in the world, and they breed in large dense colonies on small islands in the south Pacific and south Atlantic Oceans, mainly around New Zealand, the Falkland Islands, Tierra del Fuego, and in the Auckland Islands and Phillip Island off Norfolk Island. They are used as a food source for humans in Australia and New Zealand, with the New Zealand harvest estimated at 250,000 birds per year; in New Zealand, sooty shearwaters are important seabirds both culturally and ecologically. Considering the public health significance, the implications of our study may extend beyond our two study sites.”
The study also detected E. rhusiopathiae in mottled petrel and Cook’s petrel.

“Mottled petrels only breed on the Snares, Whenua Hou, and Big South Cape Island, with small colonies on other islands around Stewart Island and in Fiordland in southern New Zealand. Te Hauturu-o-Toi and Whenua Hou are the only breeding grounds for Cook’s petrels. We detected E. rhusiopathiae in Cook’s Petrels from Te Hauturu-o-Toi, but not in those from Whenua Hou. However, fewer Cook’s Petrels were sampled in Whenua Hou, and more samples would be needed to study the presence of this bacterium in that island.”
“The species-specific prevalence was higher in Cook’s petrels (13.5%) compared with that of the sooty shearwater (5.9%) and mottled petrels (2.8%). However, Cook’s petrels represent the bulk of the dead bird sample (84.0%) from Te Hauturu-o-Toi. Given this species bias in our opportunistic sampling, and that our study was limited to only two islands, we cannot make any accurate conclusions about the wider prevalence of E. rhusiopathiae in seabirds or the diversity of species affected. However, our results suggest wider studies are warranted, not only to understand the role of the seabirds for island conservation management but also to understand the effect of this infection on the seabird species.”
More research is also needed into birds carrying nonlethal infections of the bacterium, but first we need better ways to detect bacteria in blood samples.
“In acute and lethal infections, E. rhusiopathiae bacteria will be present in high numbers in bone marrow (and in blood), but the birds only survive in this state for 2–3 days. To understand the role of seabirds as reservoirs, we need to be able to identify live birds that are carrying nonlethal infections. These birds are likely to have far fewer organisms in their blood; therefore, examination of blood samples from live birds was expected to produce lower prevalence estimates than that of the bone marrow samples from dead birds and, indeed, only 1/50 mottled petrels were positive. To understand the role and prevalence of live birds that are carrying this infection, there is a need to validate sensitive techniques that can detect and quantify low numbers of organisms in blood.”
Another question is how the bacterium gets from seabird to kākāpō.
“These birds share the same environment with endangered native birds, and there is evidence of direct contact between those species, providing possible routes for pathogen spillover. On Whenua Hou, close encounters between Cook’s petrels and kākāpō have been recorded, with birds sharing the same nesting burrow on one occasion, whereas, in another record, a Cook’s petrel was killed by an aggressive female kākāpō during the breeding season. Further studies are required to confirm whether seabirds act as reservoirs for E. rhusiopathiae and whether spillover is occurring among species and, if so, the exact mechanism of transmission.”
“Our current hypothesis is that E. rhusiopathiae may be shed from decomposing infected seabirds’ bodies, contaminating the soil, which may then be ingested by other species during preening or feeding. The organism can survive in damp soil for weeks to months and environmental studies of the persistence of the organism on the islands could provide evidence to support this hypothesis.”
Perhaps we should also be vaccinating our kākāpō.
“Our finding of a potential reservoir of E. rhusiopathiae in these offshore islands should be considered when evaluating the need to vaccinate at-risk species, such as kākāpō and takahē. This is particularly important for translocations of young birds, which are likely to be more susceptible.”
Genome sequencing might also help us to better understand disease sources and transmission – just as it has help public health officials track transmission of various covid strains in humans.
“Investigation of the genetic structure of E. rhusiopathiae on the two islands studied and in different seabird species could improve our understanding of the possible sources or origin of this pathogen in the island ecosystem, whether one or several introduction events has occurred, and might identify the epidemiologic networks of the bacteria. Such information is important for the assessment of transmission risk between local and introduced translocated populations, or between marine and terrestrial birds.”
The full research report has been published in the Journal of Wildlife Diseases Paper: Seabirds as possible reservoirs of Erysipelothrix rhusiopathiae on islands used for conservation translocations in New Zealand (2021)

