Researchers Susan Walker, Joshua Kemp, Graeme Elliott, Corey Mosen and John Innes used 264,457 rodent tracking records collected quarterly from 23,709 tracking tunnel stations in forests across the length and breadth of New Zealand over an 18 year period from late 1999 to late 2016 to get a clearer picture of how ship rat and mouse populations relate to each other and their environment.
The results of their study have been published in Biological Invasions.
The research team used this massive data set to model what was happening in unmanaged rodent populations and find out more about what influences and limits population numbers. Their aim was to get a better understanding of the implications for native species conservation and to help conservation managers control rodent numbers more effectively.
Here’s what they found out.

“Populations of invasive ship rat and house mouse vary greatly over both time and space in New Zealand’s indigenous forests. In forests dominated by southern beech species, rat and mouse population densities are low for much of the time, but one or both rodent species can irrupt (a sudden, rapid population increase), following synchronised heavy seeding of the trees (‘masting’) once every 2–6 years. Ship rat populations show less marked temporal fluctuations in non-beech forests in warmer environments, where the availability of food resources is less strongly pulsed and studies record higher median and maximum rat densities and generally low densities of mice.”

But its not simply a matter of dividing forests into cool climate beech forests and warmer climate ‘other’. Many forests are somewhere in between the two types – masting forests with rodent irruptions and more stable non-beech forests.
“Elevation, aspect and soil variations in mountainous landscapes often give rise to steep gradients and complex mixtures of forest composition. Despite a large number of site-based studies, there is still incomplete understanding of large-scale spatial variation of forest rodent dynamics, and of how biotic interactions between rats and mice combine with spatially variable pulsed food resources and environmental factors to produce them.”
We need to know what’s going on to target predator control effectively and economically.
“Spatial variation in rodent dynamics across New Zealand’s 6.3 million hectares of indigenous forests has significant implications for conservation management because different predator control regimes are required to protect indigenous biota in different places. Both rodent species are predators of endemic birds, bats, lizards, and invertebrates and sustain larger predators such as stoats. Careful timing of large-scale rodent and stoat control is thought to be particularly important in forests with pulsed resources and irruptive rodent dynamics, where population collapses in indigenous bird species have been linked to peak rodent populations. In warmer, more continuously productive forests, unmanaged rats are routinely above damage density thresholds so timing of control may be set largely to coincide with vulnerable life stages of particular taxa (e.g. bird nesting).”
The data analysis and modelling had two key aims:
“In this paper our first aim is to differentiate and describe the distribution of the temporal dynamics of ship rats and house mice across New Zealand forests for the first time, so that conservation managers can develop effective rodent control strategies appropriate for the species they threaten in the locations where they occur. Until recently there has not been the empirical basis for a spatial typology, but we can now use a national database of records from a network of small-mammal tracking tunnels monitored by New Zealand’s Department of Conservation between 1999 and 2016. These data allowed us to fit models of ship rat and house mouse tracking rates as a function of time, environment, and management (i.e. predator control). We use predictions from these models to cluster forests into classes that have similar (but not synchronous) rodent temporal dynamics in the absence of management. We determine the environmental characteristics of the forest classes and map them.”

“Our second aim is to better understand how environmental variation and interspecific interactions combine to produce the observed spatial patterns of rodent dynamics. Previous local studies show that ship rats are rare much of the time in cooler environments such as high-elevation forests near and above the treeline, but warmer forests support relatively high densities of ship rats in most years. In contrast, mice have not been associated with any particular environmental factor. They occur in a wide variety of New Zealand habitats and environments and consistently increase in response to seedfall in beech forests, whereas ship rat increases are rarer. House mouse and ship rat distributions are described as ‘generally reciprocal’, mice are mainly found in pockets of dense ground vegetation where rats are abundant in forest, and mouse tracking indices typically increase following ship rat control.”
“In combination, these studies suggest that
(1) ship rat and house mouse are both limited by resources, but
(2) ship rats are regulated more strongly than house mice by mechanisms related to low temperature, and
(3) house mice are limited by ship rats through a biotic interaction. “

The researchers derived predictions from these hypotheses and fitted models to test them. In their analysis, they assumed rodents tracking rates were positively related to population densities (more tracking means more rodents, although not necessarily a directly linear relationship). They also assumed that all rat tracks were made by ship rats, which are more common by far than Norway rats in our forests.
They found that populations of invasive ship rats and house mice show distinct and predictable spatial patterns of temporal variation across New Zealand forests in the absence of predator management.
“Rodent dynamics differentiate along a continuum from cooler forests with generally low but infrequently irruptive and synchronous rat and mouse tracking rates, to warmer ‘continuously ratty’ forests with less synchronous and generally low mouse tracking rates. These patterns provide new insights and raise new questions about how climate, resource fluctuations, and biotic interactions drivers interact to drive rodent dynamics, with implications for rodent management to protect indigenous fauna.”
Cool temperatures were found to limit ship rat populations – but mice proved to be more hardy.
“There was clear evidence that low temperatures limit ship rat populations, and this effect was not eliminated in years of rodent irruptions in colder forest environments: at cooler sites, rats irrupted less frequently and attained lower tracking rates in irruptions. In contrast, neither median mouse tracking rates nor the magnitude or frequency of mouse irruptions increased with mean annual site temperature.”
“In cooler forest classes with irruptive rodent dynamics, all rat irruptions coincided with mouse irruptions, consistent with population increases being driven by the same resource pulse albeit not necessarily the same food items. Both house mice and ship rats breed through winter in years of mast seeding in New Zealand beech forests, and not often otherwise.”
Mouse numbers always rose following a masting event. Rat irruptions were less consistent, however, with other factors at play.
“Our new result is that forest rodent irruptions grade predictably from mouse-dominated (with rarer and smaller rat irruptions) to more consistently involving both high rat and high mouse densities as temperature increases. We do not yet understand the mechanisms behind this gradient: for example whether rats require shorter intervals or more benign conditions between mast events to maintain residual populations sufficient to initiate irruptions; or whether mice and rats are both extirpated from colder forests between masts, but mice are capable of increasing and re-invading colder forests more rapidly when resources are abundant.”
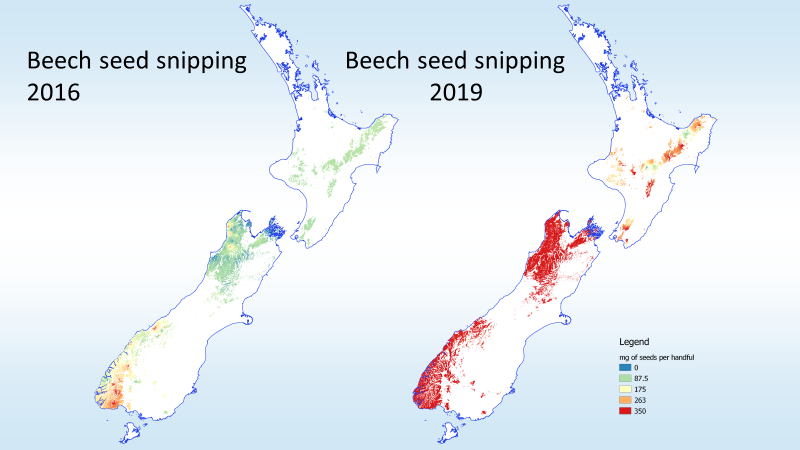
Climate change will likely make things worse, especially in southern cool-climate beech forests.
“Across forests with irruptive rodent dynamics, warmer sites had not only higher frequencies and intensities of rat irruptions, but also higher median tracking rates of ship rats. Together, these results suggest that climate warming will increase the frequency of ship rat irruptions in New Zealand’s colder forests and the elevation range of ship rats generally across forests. This in turn is likely to increase pressure on indigenous species that are vulnerable to being preyed on by ship rats and the larger predators they sustain, such as stoats and cats.”
Both the data obtained by the researchers and predictions derived from their statistical modelling showed a strong numerical or behavioural suppression of mice by ship rats. That fits with previous studies showing increases in mice following ship rat control.
“Previous modelling has suggested that mustelid predators cannot prevent or reduce the size of rodent irruptions in New Zealand’s mast-seeding forests because the intrinsic rate of increase of rodents is much higher. Our results support Innes’s (2005) suggestion that, similarly, ship rats’ intrinsic rate of increase may be insufficient to prevent or truncate mouse irruptions when both rodents are undergoing high rates of increase.”

Ferrets, stoats and cats may have an influence on rodent numbers in forests, but the researchers say its unclear by how much.
“Some have concluded that control of mustelids has little effect, but other data suggest large-scale carnivore control can cause ship rat increases.”
It all makes predator control complicated. There’s no ‘one size fits all’ solution to protecting our forests and wildlife – and what’s good for birds, may not necessarily be good for reptiles and insects which are more vulnerable to mouse predation.
“Spatially differentiated dynamics bring diverse challenges for rodent control to effectively protect New Zealand’s vulnerable native forest fauna. At one extreme are cold, rodent-irruptive forest classes where the focus of management must be on forecasting irruptions and implementing timely control in response. At the other ‘continuously ratty’ extreme, rodent suppression needs to be more frequent or even continuous, depending on conservation objectives and indigenous fauna damage thresholds. In between are extensive forest areas with intermediate median and high maximum ship rat tracking rates, which may be most challenging to manage for indigenous fauna protection. These forests may require a mixed strategy of frequent or continuous ship rat suppression that is stepped up in response to irregular peaks, which will be more difficult to predict than in floristically simpler colder forests.”
“Our results also show that suppression of ship rats is likely to release mouse populations, especially in warm forests where ship rat minimum tracking rates are highest in the absence of management. This unintended consequence has been demonstrated in other studies and could potentially negate or outweigh positive effects of ship rat suppression on mouse-vulnerable native herpetofauna and invertebrates.”
The full research paper is published in Biological Invasions but is not open access.
Spatial patterns and drivers of invasive rodent dynamics in New Zealand forests (2019)

